Lime water Formula and Chemical Name?
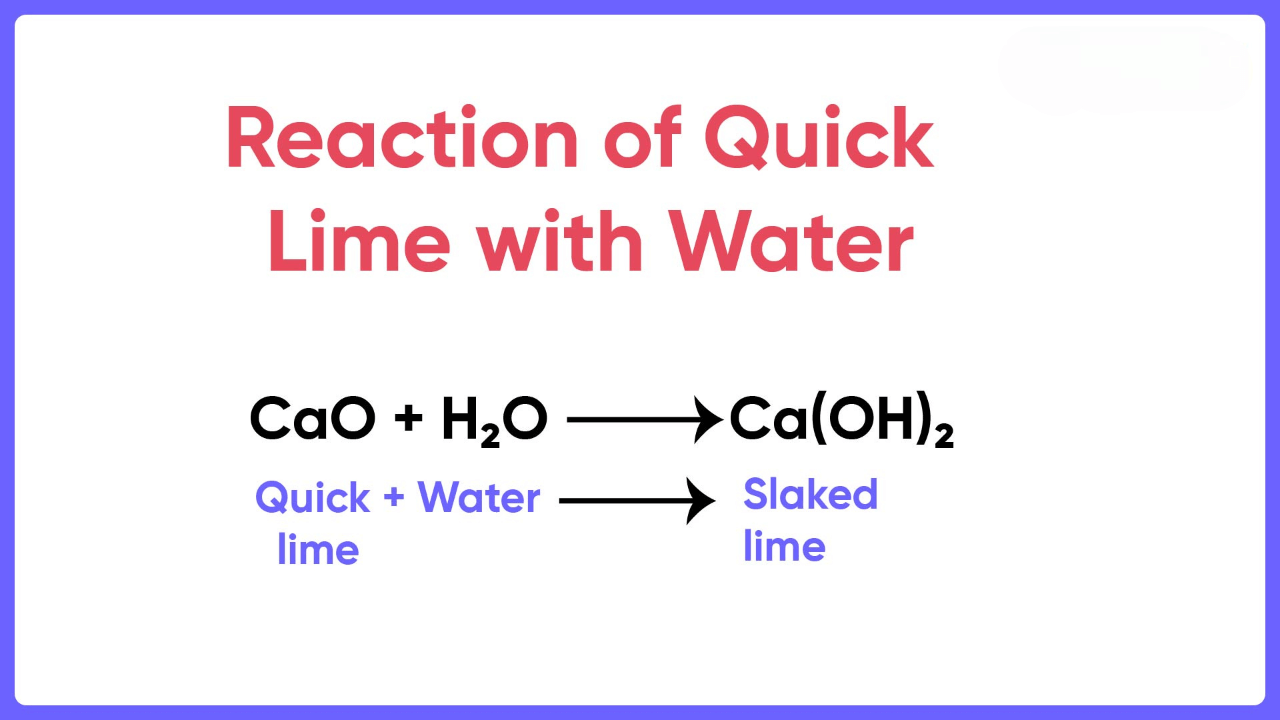
When calcium oxide is added to water, it forms a saturated solution of calcium hydroxide, which is known as lime water. Lime water can be made by adding calcium hydroxide to distilled water. The lime water formula is Ca(OH)2. We prepare Calcium hydroxide by dissolving calcium oxide (CaO), also known as quicklime, in water. The chemical reaction involved is:
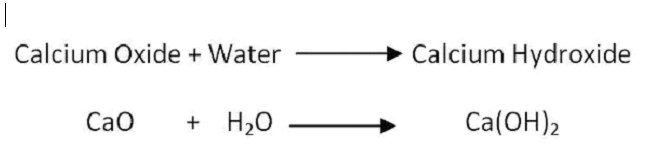
CaO + H2O → Ca(OH)2
Let us know more about what lime water is, its properties, uses, precautions, and alternatives.
What Is Lime Water
Calcium hydroxide is also known as slaked lime or hydrated lime. When calcium oxide is added to water, it forms a saturated solution of calcium hydroxide, which is known as lime water. Lime water is a clear and colorless solution that has a pH of about 12. It is commonly used in different kinds of applications, such as in the preparation of pickles, in water treatment, and as an ingredient in cement and mortar. Lime water can also be used to test for the presence of carbon dioxide gas, as it turns cloudy in the presence of CO2 due to the formation of calcium carbonate (CaCO3).
Preparation of Lime Water
Here are the steps involved in preparing lime water:
- Obtain calcium hydroxide (Ca(OH)2), also known as slaked lime or hydrated lime. Calcium hydroxide is typically sold in the form of a white powder.
- Measure the desired amount of calcium hydroxide. It will depend on the desired concentration of lime water.
- Fill a clean container made of glass like a beaker or a jar with distilled water. It is important to use pure water. If the water will be impure like tap water, impurities will affect the properties of the lime water.
- Slowly add the calcium hydroxide powder to the water while stirring constantly. The powder will start dissolving slowly. It may form lumps first but eventually, it will form a clear and saturated solution.
- Continue stirring the mixture until all of the calcium hydroxide powder has dissolved completely. The resulting solution should be clear and colorless.
- Cover the container with a lid or plastic wrap to prevent evaporation and contamination, and store the lime water in a cool, dry place until ready to use.
- Note: It is important to handle calcium hydroxide with care, as it can cause skin and eye irritation. It is recommended to wear gloves and eye protection when handling the powder and to avoid inhaling the dust.
Properties of Lime Water
Lime water is a clear, colorless, and slightly bitter-tasting solution that has several distinctive physical and chemical properties. Here are some of the key properties of lime water:
pH
Lime water is strongly basic, with a pH of approximately 12. This means it has a high concentration of hydroxide ions (OH-) and a low concentration of hydrogen ions (H+).
Density
The density of lime water varies depending on its concentration. A typical concentration of lime water is 1 gram of calcium hydroxide per liter of water, which has a density of approximately 1.08 grams per cubic centimeter (g/cm3).
Solubility
Calcium hydroxide is only slightly soluble in water, with a solubility of approximately 1.5 grams per liter of water at room temperature. This means that it can take some time for the powder to fully dissolve in water and that the resulting solution may be supersaturated.
Reactivity
Lime water is reactive with many other substances, including carbon dioxide (CO2), which causes it to turn cloudy or milky due to the formation of calcium carbonate (CaCO3) precipitate. Lime water can also react with acids to form calcium salts, and with metal ions to form insoluble hydroxides.
Taste
Lime water has a slightly bitter taste due to its alkaline pH.
Appearance
Lime water is a clear, colorless solution that may appear cloudy if it has reacted with carbon dioxide. The solution can also turn yellow if it has been exposed to light for an extended period, due to the formation of calcium salts.
Overall, the unique properties of lime water make it useful in a variety of applications, such as in food preservation, water treatment, construction, medicine, and science.
Uses of Lime Water
Lime water has a wide range of uses in various fields due to its strong alkaline properties and reactivity with other substances. Here are some of the common uses of lime water:
Laboratory experiments
Lime water is commonly used in science experiments to test for the presence of carbon dioxide gas. When carbon dioxide is bubbled through lime water, it reacts with the calcium hydroxide to form calcium carbonate, causing the lime water to turn cloudy or milky.
Water treatment
Lime water is used in water treatment to remove impurities and adjust pH levels. It can be added to water to increase the pH and cause metals and other impurities to precipitate out of the water.
Food preservation
Lime water is used as an ingredient in the preservation of foods such as pickles, which helps to maintain vegetables and prevent spoilage.
Construction
Lime water is used in construction as a component of mortar and cement. It reacts with carbon dioxide in the air to form calcium carbonate, which helps to harden and strengthen the building material.
Medicine
Lime water is used in medicine as a treatment for acid reflux and indigestion. It works by neutralizing the excess acid in the stomach and esophagus.
Agriculture
Lime water is used in agriculture to adjust soil pH levels, as it can help to neutralize acidic soil and improve crop yields.
Overall, lime water is a versatile substance that has many practical uses in a variety of fields.
Safety Precautions When Handling Lime Water
Lime water is a strong base and can be hazardous if not handled properly. Here are some safety precautions to follow when handling lime water:
Wear protective gear
When handling lime water, always wear protective gear such as gloves, safety glasses, or goggles, and a lab coat or apron to protect your skin and eyes from contact with the solution.
Use caution when handling the powder
Calcium hydroxide powder, which is used to make lime water, can irritate the skin and eyes. Avoid breathing in the dust and do not touch the powder with bare hands.
Mix the solution in a well-ventilated area
When preparing lime water, work in a well-ventilated area to avoid inhaling any fumes that may be produced.
Avoid contact with acids
Lime water is a strong base and should not be mixed with acids, as it can produce heat and cause spattering.
Store lime water properly
After preparing lime water, store it in a sealed container in a cool, dry place away from direct sunlight.
Dispose of waste properly
Any unused or excess lime water should be disposed of according to local regulations.
Overall, it is important to handle lime water with care and follow all safety precautions to prevent injury or damage to equipment. However, it is important to handle it with care due to its corrosive properties and potential for skin and eye irritation. If you experience any skin or eye irritation, rinse the affected area with plenty of water and seek medical attention if necessary.
Alternatives to Lime Water
There are a few alternatives to lime water, depending on the specific application. Here are some common alternatives:
Sodium hydroxide (caustic soda)
Sodium hydroxide is a strong base that is commonly used as an alternative to lime water in applications such as water treatment and soap making.
Potassium hydroxide (caustic potash)
Potassium hydroxide is another strong base that can be used as an alternative to lime water in some applications, such as in the production of biodiesel.
Magnesium hydroxide
Magnesium hydroxide is a mild base that can be used in water in some medical applications, such as for the treatment of acid reflux and heartburn.
Calcium carbonate
Calcium carbonate is a chemical compound that can be used in applications, such as in the production of cement and as a dietary supplement.
⦁ Sodium bicarbonate (baking soda)
Sodium bicarbonate is a mild base that can be used in some medical applications, such as for the treatment of indigestion and heartburn.
It is important to note that each of these alternatives has its unique properties and may not be suitable for all applications. It is always best to consult with a professional or reference material specific to your application before using an alternative to lime water.